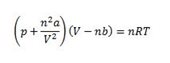
Generalized compressibility factor Z diagram.
where:
- a is the interaction energy between molecules;
- b is the occupied volume by the molecules.
This equation gives a much better prediction of real gas behaviour in practice. Each gas (or mixture) has different a and b coefficients. When the molecules do not interact (a=0) and do not occupy space (b=0), the result is again the ideal gas law.
The ideal gas law – Benedict-Webb-Rubin equation
The equation of state used in Fluidat is based on a more advanced virial equation of state (an expression of a system derived from statistical mechanics, usually describing a system in equilibrium as a power series of particle interactions). It is called the Benedict-Webb-Rubin equation, named after the three researchers (M. Benedict, G.B. Webb and L.C. Rubin) working at the research laboratory of M. W. Kellogg Limited who determined the model. From this equation of state the non-ideal behaviour of fluids can be derived, a required input for the calculation of physical properties like:
- density
- heat capacity
- thermal conduction
- viscosity
- and vapour pressure
The Benedict-Webb-Rubin equations are calculated using intrinsic properties, like molar mass, critical properties, polarity, acentric factor and other parameters. These intrinsic properties characterize the fluid, including effects like compressibility, variable specific heat capacity, and Van der Waals forces. These properties will influence the physical properties of a fluid.